The lives of close to 600 million people worldwide are deeply affected by mitral insufficiency and urinary incontinence. Those with mitral insufficiency must undergo major surgery to avoid all cardiac problems, or even simply to survive. As for urinary incontinence, sufferers change their social life due to leaking, and have to make do with solutions that are difficult to implant and use. All expect easier solutions from modern medicine.
Which is what Affluent offers, with three leading-edge solutions: minimally invasive, adjustable and modelled on the human anatomy. Kalios™ and Epygon treat mitral insufficiency, and Artus treats urinary incontinence.
At the clinical phase today, each of these innovations will be available starting in 2027.
Solutions at the Clinical Phase of Study, and Soon Accessible for Everyone

Structural heart
Kalios™
Mitral valve repair

Affluent Medical’s objective is to submit a De Novo application with current clinical data at the end of 2025 to be followed by commercial launch, subject to Edwards’ decisions, with whom Affluent Medical has signed several agreements related to its structural heart products and technologies.

Structural heart
Epygon
Mitral valve replacement
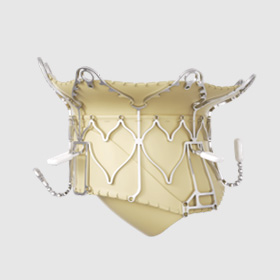
In 2024, the company began a collaboration with Prof. Mohammad Sarraf, MD, interventional cardiologist at the Mayo Clinic in the US, to evaluate the benefits of the biomimetic design of the Epygon valve that aims to replicate the natural physiology of the native mitral valve to enable patients to recover good cardiac function more quickly.
Affluent Medical accelerated patient screening with the goal to implant up to ten patients to complete the pilot phase.

Urology
Artus
Urinary incontinence

In January 2025, the Company announced the completion of the enrollment for the pilot phase of the European multicenter clinical study in humans with the successful 10th minimally invasive implantation in men.
To date, 100% of devices having been successfully activated and the safety profile remains positive. The pivotal phase, aiming to validate the device’s performance in reducing incontinence in several dozen patients, is scheduled to begin in Q2 2025.
With women representing about 80% of patients suffering from urinary incontinence, the Company will submit the dossier in order to begin a pilot study in women in the first half of 2025.