Innovative Solutions, that are Minimally Invasive, Biomimetic and Adjustable.
Solutions at the Clinical Phase of Study, and Soon Accessible for Everyone
All of Affluent’s solutions are currently at the clinical phase of study. If tests confirm their viability, they can be offered to patients starting in 2025, starting with Kalios™.

Structural heart
Kalios™
Mitral valve repair

Affluent Medical’s objective is to submit a De Novo application with current clinical data at the end of 2025 to be followed by commercial launch, subject to Edwards’ decisions, with whom Affluent Medical has signed several agreements related to its structural heart products and technologies.

Structural heart
Epygon
Mitral valve replacement
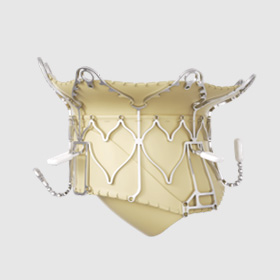
In 2024, the company began a collaboration with Prof. Mohammad Sarraf, MD, interventional cardiologist at the Mayo Clinic in the US, to evaluate the benefits of the biomimetic design of the Epygon valve that aims to replicate the natural physiology of the native mitral valve to enable patients to recover good cardiac function more quickly.
Affluent Medical accelerated patient screening with the goal to implant up to ten patients to complete the pilot phase.

Urology
Artus
Urinary incontinence

In January 2025, the Company announced the completion of the enrollment for the pilot phase of the European multicenter clinical study in humans with the successful 10th minimally invasive implantation in men.
To date, 100% of devices having been successfully activated and the safety profile remains positive. The pivotal phase, aiming to validate the device’s performance in reducing incontinence in several dozen patients, is scheduled to begin in Q2 2025.
With women representing about 80% of patients suffering from urinary incontinence, the Company will submit the dossier in order to begin a pilot study in women in the first half of 2025.
360°-Protected Innovations right up to 2041
Apart from the patents that protect each product, Affluent has also protected all the innovations that compose them: materials, technologies, manufacturing processes, etc.